Larrin
Gold Member
- Joined
- Jan 17, 2004
- Messages
- 5,056
The speed at which you have to quench a steel to obtain full hardness or full martensite is known as hardenability. Hardenability is not a measure of how hard the steel can get but simply how fast it must be cooled to get there. Steels with different levels of hardenability are sometimes categorized into the type of quench required, such as water hardening, oil hardening, or air hardening.
We can measure hardenability in a few different ways. One way is with a Continuous Cooling Transformation (CCT) diagram. The steels are first austenitized by heating to high temperature and holding for the proscribed time, and then cooled at different rates. In-situ measurements are recorded such as dilatometry to determine the temperatures at which the steel transforms to other phases. Often the final samples are also measured for hardness and observed with metallography. An example CCT curve for a "eutectoid steel", probably 1075 or 1080, is shown below:

A water quench cools the steel very rapidly, within a few seconds, leading to full martensite. The "full anneal" cools very slowly and passes through those lines at top referring to the pearlite transformation start and finish. The "critical cooling rate" shows the cooling rate required to pass in front of the "nose" without any pearlite formation. The oil quench is not fast enough to avoid pearlite formation so this steel would be classified as a water quenching steel.
Certain alloying elements can be added to enhance hardenability and allow slower quench rates. These alloying elements push the "nose" of the CCT curve to the right, ie at longer times. The following diagram shows the relative effect of a few of the important elements:

So Molybdenum (Mo) is a very effective hardenability element, followed by Mn and Cr, with Ni and Si having relatively small effects on hardenability.
O1 is an oil hardening steel with additions of Mn and Cr for added hardenability to allow an oil quench. You can see its CCT curve below:

Where the "eutectoid" steel with low hardenability additions required a quench lasting around 20 seconds, O1 looks like it can be quenched at a rate almost 10x slower than that, and therefore allows oil hardening rather than water.
If even more alloy is added than an air hardening steel is obtained, such as Vanadis 4 Extra:

Vanadis 4 Extra can be cooled even more slowly, and therefore can allow hardening in air instead of water or oil. However, one interesting thing is that full hardness is shown even for the cooling curves labeled 3-5 which pass through the carbide section of the CCT curve. So obtaining full hardness may not be a guarantee of full martensite. The carbides may not be desirable for mechanical properties, or with a stainless steel for corrosion resistance.
Another method for measuring hardenability is the Jominy test. A cylinder of steel is heated evenly at the austenitizing temperature, and then cooled on one end with water:
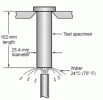
The end sprayed with water is cooled very rapidly, and the cooling rate is progressively slower the further you go from the water cooled end. Then hardness can be measured along the bar:


These Jominy tests can give an indication of what thickness of material can be fully hardened, as they provide quick information on thickness vs hardness. After all, if quenching a very thick piece of steel, the core will cool much more slowly than the surface. With an air hardening steel the core is likely to still form full martensite. But with a water quenching steel, the core may not fully harden even with thicknesses relevant to knives. These Jominy tests may also be used to compare relative hardenability of different steels:

This gives an obvious example of how the different alloying elements directly affect hardenability. Also, all of the steels reached the same hardness, apart from 1040, showing that hardenability and peak hardness are two different concepts.
There are several other things that can affect hardenability, and I will cover two of them. With high carbon steels there are carbides that are intentionally left in the steel during austenitizing. With higher temperatures, more of these carbides dissolve so that more alloy is in solution. For example, as a stainless steel is heated to higher austenitizing temperatures more of its chromium carbides dissolve leaving more carbon and chromium in solution. That carbon and chromium increases the hardenability. So in general hardenability is increased with higher austenitizing temperatures.
One more important factor is grain size. Grain boundaries are high energy areas that act as nucleation sites for the transformation phases. So if the carbides or pearlite nucleate they are likely to do so on the austenite grain boundaries. With finer grain size there is more boundary area for nucleation. So finer grain size leads to poorer hardenability:

(A bigger number of ASTM grain size refers to a smaller grain size.) Overaustenitizing leads to grain growth, so again, a higher austenitizing temperature can lead to an improvement in hardenability.
We can measure hardenability in a few different ways. One way is with a Continuous Cooling Transformation (CCT) diagram. The steels are first austenitized by heating to high temperature and holding for the proscribed time, and then cooled at different rates. In-situ measurements are recorded such as dilatometry to determine the temperatures at which the steel transforms to other phases. Often the final samples are also measured for hardness and observed with metallography. An example CCT curve for a "eutectoid steel", probably 1075 or 1080, is shown below:

A water quench cools the steel very rapidly, within a few seconds, leading to full martensite. The "full anneal" cools very slowly and passes through those lines at top referring to the pearlite transformation start and finish. The "critical cooling rate" shows the cooling rate required to pass in front of the "nose" without any pearlite formation. The oil quench is not fast enough to avoid pearlite formation so this steel would be classified as a water quenching steel.
Certain alloying elements can be added to enhance hardenability and allow slower quench rates. These alloying elements push the "nose" of the CCT curve to the right, ie at longer times. The following diagram shows the relative effect of a few of the important elements:

So Molybdenum (Mo) is a very effective hardenability element, followed by Mn and Cr, with Ni and Si having relatively small effects on hardenability.
O1 is an oil hardening steel with additions of Mn and Cr for added hardenability to allow an oil quench. You can see its CCT curve below:

Where the "eutectoid" steel with low hardenability additions required a quench lasting around 20 seconds, O1 looks like it can be quenched at a rate almost 10x slower than that, and therefore allows oil hardening rather than water.
If even more alloy is added than an air hardening steel is obtained, such as Vanadis 4 Extra:

Vanadis 4 Extra can be cooled even more slowly, and therefore can allow hardening in air instead of water or oil. However, one interesting thing is that full hardness is shown even for the cooling curves labeled 3-5 which pass through the carbide section of the CCT curve. So obtaining full hardness may not be a guarantee of full martensite. The carbides may not be desirable for mechanical properties, or with a stainless steel for corrosion resistance.
Another method for measuring hardenability is the Jominy test. A cylinder of steel is heated evenly at the austenitizing temperature, and then cooled on one end with water:

The end sprayed with water is cooled very rapidly, and the cooling rate is progressively slower the further you go from the water cooled end. Then hardness can be measured along the bar:


These Jominy tests can give an indication of what thickness of material can be fully hardened, as they provide quick information on thickness vs hardness. After all, if quenching a very thick piece of steel, the core will cool much more slowly than the surface. With an air hardening steel the core is likely to still form full martensite. But with a water quenching steel, the core may not fully harden even with thicknesses relevant to knives. These Jominy tests may also be used to compare relative hardenability of different steels:

This gives an obvious example of how the different alloying elements directly affect hardenability. Also, all of the steels reached the same hardness, apart from 1040, showing that hardenability and peak hardness are two different concepts.
There are several other things that can affect hardenability, and I will cover two of them. With high carbon steels there are carbides that are intentionally left in the steel during austenitizing. With higher temperatures, more of these carbides dissolve so that more alloy is in solution. For example, as a stainless steel is heated to higher austenitizing temperatures more of its chromium carbides dissolve leaving more carbon and chromium in solution. That carbon and chromium increases the hardenability. So in general hardenability is increased with higher austenitizing temperatures.
One more important factor is grain size. Grain boundaries are high energy areas that act as nucleation sites for the transformation phases. So if the carbides or pearlite nucleate they are likely to do so on the austenite grain boundaries. With finer grain size there is more boundary area for nucleation. So finer grain size leads to poorer hardenability:

(A bigger number of ASTM grain size refers to a smaller grain size.) Overaustenitizing leads to grain growth, so again, a higher austenitizing temperature can lead to an improvement in hardenability.