... I have tested extremely sharp blades and in that case there is no room for carbides on the thin cutting edge.
...the hazelnut chocolate demonstration shows it clearly; when the target is ultimate sharpness, carbide-rich alloy steels are not necessarily better than plain carbon steels.
While I agree that carbide-rich alloys may not be preferred to low-alloy steels for achieving high sharpness (depending on HT), I am often struck by the need to re-iterate that
"when the target is ultimate sharpness" you want ONLY carbide in the apex.
As Luong correctly points out,
bending strength accounts for the vast majority of edge performance in cutting tasks - i.e. the
stiffer apex-material takes the keener edge. It is precisely for this reason that the finest-edged cutting tools are made from carbide hard-metals, not from steel at all!!! The finest steel apices on fine-grained steel are commonly ~0.5 um, while the working apex diameter of WC-Co and diamond and obsidian edges are commonly 0.05 - 0.005 um, some
10x - 100x sharper! The "pure" carbide materials can achieve this because of their strength (hardness). Increasing Rockwell hardness in steel brings it closer and closer to the performance of these materials. However, where these carbide hard-metals fail is macro-toughness - once you overcome that resistance to bending, they fracture readily, and the thinner the bevel-angle (geometry) BEHIND the apex, the weaker (cubic) the material is with regard to resisting lateral stress.
As Luong previously pointed out, there is a complex relationship between the carbides and the steel matrix "binder" in which they are embedded. The more carbide that you have embedded in the very apex of the cutting edge,
the SHARPER your blade can be made!!! But the ability to achieve that edge, and the durability of that edge in your specific use, as well as the utility of even having an edge that is 10x - 100x sharper than steel can achieve, coupled with the expense of replacing the blade when it fails, all these factors contribute to the impracticality of such tools in everyday life.
I just want to be sure to avoid confusion in these conversations. When we are talking about a blade as "sharp", it is helpful to give an actual measurement, e.g. apex diameter, to put the concept into context. A 12C27 blade sharpened to 0.5 um apex diameter is "sharp" for a steel, but it is flat "dull" compared to blades achieving 0.05 and 0.005 um diameters. 12C27 (one of Sanvik's very fine-grained steels) cannot achieve 0.005 um with any level of stability because it is
far too soft. And just to play with one's head, recognize that appropriately sharpened D2 with massive carbides in the apex
could achieve that sharper edge specifically where those carbides reside.
Carbides are not only able to achieve superior sharpness, but they are also less susceptible to heat (and generate less heat during cutting) and, as has been mentioned and often comes up in knife discussions, they are more resistance to abrasion. These features make them ideal for drill-bits and saw-teeth and milling-tools, stamps, lathes, they make them ideal for
the tools you use to cut and sharpen steel blades.
The trouble with carbide is, again, that most people could neither achieve nor appreciate the level of sharpness attainable with such materials when what they REALLY want is a TOUGH tool that is very thin
behind the edge while simply acceptably sharp at the apex. I am happy with a knife that can whittle a hair though even that is excessive for shaving, and of what use is such a tool to most people if it is sharp enough to trace out individual cells from a slice of plant stem but is easily fractured if made into a blade 4" long with a 5-dps primary bevel?
I really appreciate what Luong does because he is pushing for the HIGHEST possible Rc hardness, to bring his blades as close to the strength of carbide hard-metals as he can, but while simultaneously demanding that they endure severe impact force and edge-steering.

The steel industry has been working toward more and more carbide-rich steel in order to improve tool-performance while trying to maintain the toughness that steel is known for. If they didn't care about toughness, they wouldn't be working on refining carbides. While on the other end, hard-metals are almost entirely carbide but the industry is working on making them tougher to be more like steel without sacrificing the strength that carbide is known for

When it comes to razor-blades and shaving, applications where a fine edge of very low edge-diameter is required but relatively stout behind-the-edge geometry may be preferred (e.g. 0.05 um diameter with a convex edge starting from around 45-dps) and impact-toughness is unimportant, then they very best material to use is one with the near-uniform superior hardness of carbide. Steel is acceptable but inferior.
When it comes to saw-blades which require moderate impact toughness and a very fine edge of low diameter resistant to heat and abrasion, but where again a very thin behind-the-edge geometry is not really required, again the carbide is preferred.
But when it comes to a long knife subject to bending and impacts and irregular attempts at re-profiling or changing the geometry, where a fine edge of relatively thick diameter is all that is required, THEN we prefer steel because it is stronger than plain old iron and more ductile than plain carbon - mix them together and Tah-Dah!! Magic

To get back to the OP, a "carbide rich" steel suffers from rough edges
NOT because of the carbides but rather because of the binder, i.e. the steel around them. Improve the bind between carbide and matrix, and you improve your edge. Carbides "tear-out" is not the fault of the carbides, it is the fault of the binder not holding them. This can be mitigated in large part by geometry, but it can also be mitigated by increasing carbide content WELL above the <20% level found in steels. When you have no or fewer or smaller carbides in the apex, you can make a finely finished apex
but of inferior diameter and longevity if your ultimate goal was the sharpest possible edge.







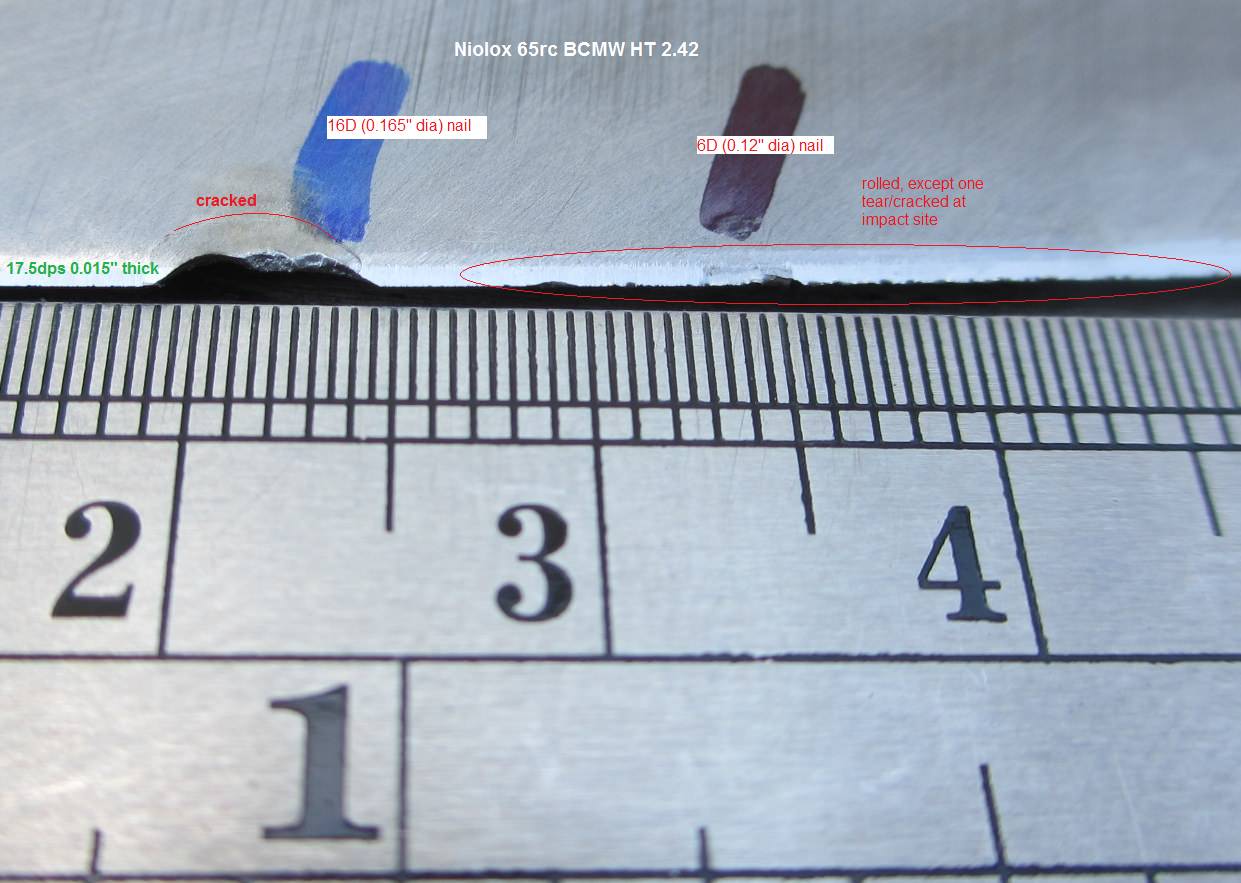
![IMG]](/proxy.php?image=http%3A%2F%2F%5BURL%3D%27http%3A%2F%2Fimages41.fotki.com%2Fv1647%2Fphotos%2F4%2F3821034%2F14371630%2FUNADJUSTEDNONRAW_thumb_33d-vi.jpg%255B%27%5Dhttp%3A%2F%2Fimages41.fotki.com%2Fv1647%2Fphotos%2F4%2F3821034%2F14371630%2FUNADJUSTEDNONRAW_thumb_33d-vi.jpg%5B%5B%2FURL%5D%2FIMG%5D&hash=b658a27893343061fbe5f5d0ce9db1db)
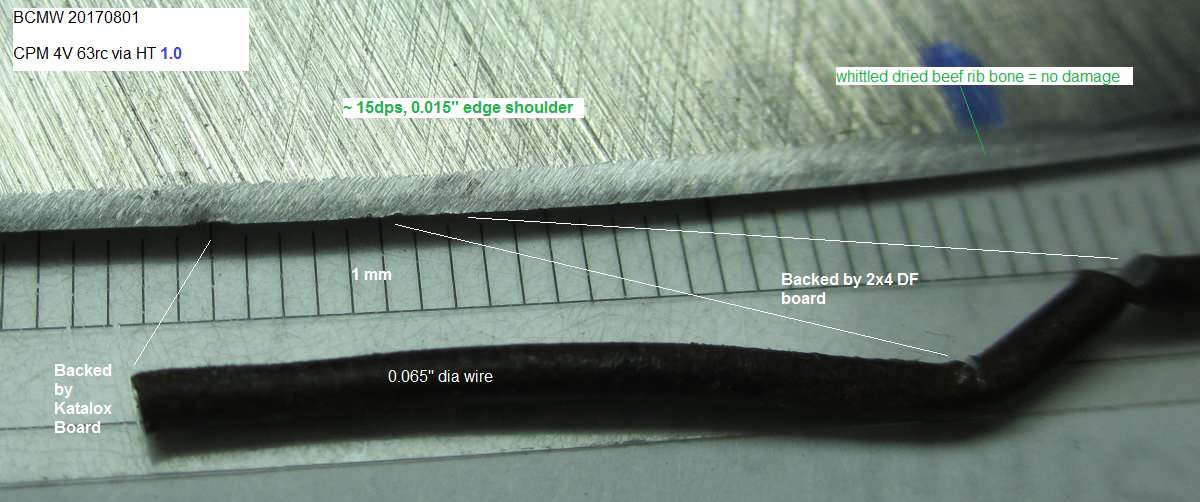
 Hopefully, you didn't fixed these edges yet because it would be informative to see how their apexes after cut to about 1/3 diameter (then pull out) of the bailing wire. Essentially testing apex stability - and of course, macro pics of edges afterward. Please don't try to peen 4V ripple back, just sharpen it out, where affected area will be weaker and still better than a large chip
Hopefully, you didn't fixed these edges yet because it would be informative to see how their apexes after cut to about 1/3 diameter (then pull out) of the bailing wire. Essentially testing apex stability - and of course, macro pics of edges afterward. Please don't try to peen 4V ripple back, just sharpen it out, where affected area will be weaker and still better than a large chip