Larrin
Knifemaker / Craftsman / Service Provider
- Joined
- Jan 17, 2004
- Messages
- 4,845
Do I need liquid nitrogen or is dry ice good enough? Short answer: Go as cold as you can to minimize retained austenite. A freezer is better than room temperature. Do you want to know why? Then read below...
At high temperature we form the nonmagnetic phase austenite and dissolve carbides so that we have carbon in solution. During rapid cooling we form martensite laths or plates. An example of what those look like can be seen here:

The white grains are the austenite and those feather looking things are the martensite laths. Martensite forms based on temperature rather than time, so a reduction in temperature is required to form further martensite.

You can see a fun video of martensite formation in action here:
"Retained austenite" is the austenite that does not transform to martensite. You can see a whole bunch of it in those micrographs I linked before where only a small fraction of martensite is present. In knife steels we usually have a significantly smaller amount though it's possible to get a pretty good amount of it, perhaps up to 50% with a richly alloyed steel that was austenitized too high. But we are getting ahead of ourselves; why do some steels need to go below room temperature and others do not? That is all based on the martensite finish temperature, which is alloy dependent. Furthermore, the composition of the austenite changes based on the austenitizing temperature chosen, because more carbides are dissolved at higher temperature which enriches the austenite with further carbon, chromium, or other elements. Retained austenite measurements can be performed with a few methods such as X-ray diffraction to determine if any retained austenite is present, but these values are not always known or are unlikely to be available to the average knifemaker.
Certain alloying elements like carbon are especially effective at reducing the martensite start and martensite finish temperatures, as can be seen below:

Can we estimate retained austenite? Several empirical equations have been developed to predict the martensite start temperature, which will also control in part our martensite finish. Here is a popular one from Andrews:
Ms (Celsius) = 539 - 423*C - 30.4*Mn - 12.1*Cr - 7.5*Mo - 7.5*Si
Martensite finish can also be estimated with the Koistinen-Marburger equation:
Martensite fraction = 1 - exp(-alpha*(Ms-T))
where alpha is a constant, originally calculated to be 0.011 but is dependent on composition, Ms is the martensite start temperature and T is the temperature of interest, such as room temperature, all in Celsius.
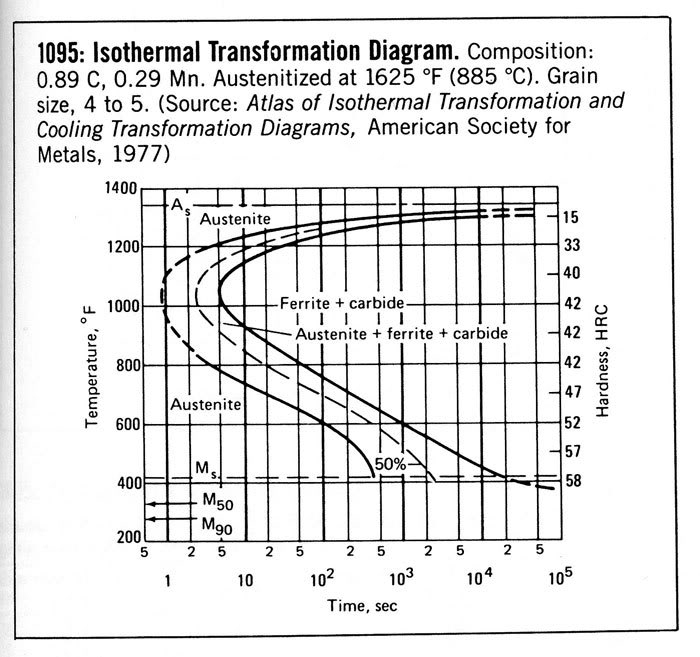
Therefore to determine if a steel needs to be cooled below room temperature to fully transform, the composition of the austenite must be measured or at least calculated, such as with Thermodynamic software. For example, a fully austenitized 1020 steel with 0.5% Mn and 0.4% Si would have 1.1% retained austenite at room temperature, 0.3% at dry ice, and 0.1% at liquid nitrogen temperature. This is using the 0.011 alpha parameter. 3V steel austenitized at 1960F would have 6.7, 2.2, and 0.6, respectively, all relatively low amounts. 1095 steel fully austenitized, however, would have 32% retained austenite! With lower austenitizing temperatures there would be less, of course, but its high carbon content means it usually has some retained austenite. Just because 1095 is a simple carbon steel does not mean that retained austenite is not a factor.
Why transform retained austenite to martensite at all? Well one major reason is for higher hardness, as austenite is much softer than martensite. You can see the effect in this chart below:

Once past a certain amount of carbon the hardness starts to level off or even decrease because of the effect of retained austenite. Retained austenite, however, can improve impact toughness so it is not always universally bad. Some knifemakers are concerned that if the austenite transforms during use that it will be untempered and therefore act as an easy site for fracture. Those concerns could very well be well founded. This is the reason that multiple tempers are recommended for high alloy steels, because tempering can also promote the transformation of austenite and therefore further tempers are required to temper the new martensite formed from the previous temper.
Well this was too much information in too small a space but I did my best.
At high temperature we form the nonmagnetic phase austenite and dissolve carbides so that we have carbon in solution. During rapid cooling we form martensite laths or plates. An example of what those look like can be seen here:

The white grains are the austenite and those feather looking things are the martensite laths. Martensite forms based on temperature rather than time, so a reduction in temperature is required to form further martensite.

You can see a fun video of martensite formation in action here:
"Retained austenite" is the austenite that does not transform to martensite. You can see a whole bunch of it in those micrographs I linked before where only a small fraction of martensite is present. In knife steels we usually have a significantly smaller amount though it's possible to get a pretty good amount of it, perhaps up to 50% with a richly alloyed steel that was austenitized too high. But we are getting ahead of ourselves; why do some steels need to go below room temperature and others do not? That is all based on the martensite finish temperature, which is alloy dependent. Furthermore, the composition of the austenite changes based on the austenitizing temperature chosen, because more carbides are dissolved at higher temperature which enriches the austenite with further carbon, chromium, or other elements. Retained austenite measurements can be performed with a few methods such as X-ray diffraction to determine if any retained austenite is present, but these values are not always known or are unlikely to be available to the average knifemaker.
Certain alloying elements like carbon are especially effective at reducing the martensite start and martensite finish temperatures, as can be seen below:

Can we estimate retained austenite? Several empirical equations have been developed to predict the martensite start temperature, which will also control in part our martensite finish. Here is a popular one from Andrews:
Ms (Celsius) = 539 - 423*C - 30.4*Mn - 12.1*Cr - 7.5*Mo - 7.5*Si
Martensite finish can also be estimated with the Koistinen-Marburger equation:
Martensite fraction = 1 - exp(-alpha*(Ms-T))
where alpha is a constant, originally calculated to be 0.011 but is dependent on composition, Ms is the martensite start temperature and T is the temperature of interest, such as room temperature, all in Celsius.
Therefore to determine if a steel needs to be cooled below room temperature to fully transform, the composition of the austenite must be measured or at least calculated, such as with Thermodynamic software. For example, a fully austenitized 1020 steel with 0.5% Mn and 0.4% Si would have 1.1% retained austenite at room temperature, 0.3% at dry ice, and 0.1% at liquid nitrogen temperature. This is using the 0.011 alpha parameter. 3V steel austenitized at 1960F would have 6.7, 2.2, and 0.6, respectively, all relatively low amounts. 1095 steel fully austenitized, however, would have 32% retained austenite! With lower austenitizing temperatures there would be less, of course, but its high carbon content means it usually has some retained austenite. Just because 1095 is a simple carbon steel does not mean that retained austenite is not a factor.
Why transform retained austenite to martensite at all? Well one major reason is for higher hardness, as austenite is much softer than martensite. You can see the effect in this chart below:

Once past a certain amount of carbon the hardness starts to level off or even decrease because of the effect of retained austenite. Retained austenite, however, can improve impact toughness so it is not always universally bad. Some knifemakers are concerned that if the austenite transforms during use that it will be untempered and therefore act as an easy site for fracture. Those concerns could very well be well founded. This is the reason that multiple tempers are recommended for high alloy steels, because tempering can also promote the transformation of austenite and therefore further tempers are required to temper the new martensite formed from the previous temper.
Well this was too much information in too small a space but I did my best.
Last edited: