ok .... let me start taking a stab at talking about how heat is moving around inside the chamber of the oven. First though (I think this is pertinent, but maybe is just be nerding out.....) .... lets take a quick look at just what energy (which is the same as "heat") and Temperature are.
Energy ..... no one has the slightest idea just what it is. We know how to **describe** it and how it changes (i.e. "potential energy", "kinetic energy", "radiative energy" (photons)), but nobody really knows what it is. It is even weirder in that its existence/magnitude is relative. ride along next to a bullet traveling from a gun at the same speed as the bullet, and, for you, the bullet has no kinetic energy and can do no harm to you at all. If you are however, going at the same "speed" as the gun that fired the bullet, well to you that bullet has a LOT of kinetic energy. Things get even weirder from there .... so for now lets just say we do not know what energy is, except that it can move around and change how it appears.
Temperature, though, is actually very well defined. It is motion - pure and simple. For a gas, the faster (i.e. kinetic energy) the atoms that make up the gas fly around from place to place the "hotter" it is considered to be. add energy to the atoms, they increase their kinetic energy, and we say that it is "hotter" (how those velocities of the atoms change with temperature is described by the "Maxwell-Boltzmann distribution".
For solids and liquids, temperature is defined by the number of ways the atoms that make up the molecules can vibrate. For example, a simple molecule like oxygen or nitrogen has only two atoms. The ONLY way those atoms can vibrate is to go in-and-out relative to each other (like a simple spring expanding and contracting):
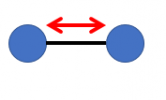
without going into detail, the MAGNITUDE of how big that vibration is is NOT continuous, but takes place in discrete steps (believe it or not .... it is this point that Einstein got his one and only Nobel prize for .... NOT for Relativity theory). so .... when the molecule contains NOT a lot of energy, it can only vibrate in-out in one small motion. Put more energy into it, and it can vibrate in-out to bigger and bigger extents (again, in discrete steps of changes) .... and as it gains the ability to vibrate to greater and greater extents, we say it is "hotter". How do we put energy into it? well, we can bump it physically (like swinging a baseball bat through the air), or (a
nd this is the important part here) a photon of light can hit the molecule and be "absorbed" by the molecule ..... giving the energy the photon carried to the molecule and giving it more ways to vibrate. Again - this is important for us here .... the opposite can also happen: if the molecule is vibrating with higher and higher amounts of movement .... the very act of that motion causes photons to be
given off by the molecule (this is exactly how a transmitting radio antenna transmits a signal). by giving off that photon, the molecule loses energy, and we say it has "cooled off" somewhat.
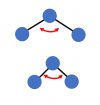
Other more complicated molecules have many more ways in which they can vibrate: a three-atom molecule like water has three ways it can vibrate. a simple symmetrical in-out of the two atoms on the "outside" of the molecule:
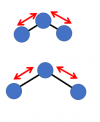
or another in-out motion, but this one being not symmetrical (more of a back-and-forth than an in-out motion)"
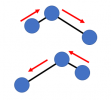
and finally a motion kind of like a "flexure":
Each of these motions are independent of each other, and it takes more and more energy to activate one and then the other, and then the last. For this reason, this more complicated molecule
has a higher heat capacity than does a simple two atom molecule like oxygen or nitrogen. And also, like in the case of the two atom molecule, each of these independent motions can take place to lesser or greater extents - not continuously, but in discrete steps. And again, each of these motions can be activated by a photon (of light), and when more and more of these modes of vibration are activated .... we say the molecule has become "hotter". And also as with the two-atom molecule, once one of these more vigorous modes of vibration are activated (the thing gets hotter), t
hen a photon will be given off just as an antenna gives off photons, and we say the thing has cooled off a little bit.
it is because of these photons given off by vibrating molecules in something that a "hot" material glows. When something is not all that hot (in the scheme of things), only a few of the available vibrations are activated (lower energy ones at that) .... and the things glows "red" (only taking up and giving off "infrared" and "red" photons). As the thing gets hotter, more and more different types of vibration are activated, and more and more photons of different energies are taken up and given off .... and we say the thing is "white hot" (white being a mixture of many different colors).
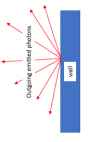
But the main point here behind how the inside of an oven works is that the photons flying around inside the oven do NOT just "bounce" off the walls - they are absorbed by the molecules in the walls, and then different photons are re-radiated off into the chamber. I know all that seems like a nerdy aside - but it really is not. Please let me know if it makes sense???
Nerdy aside 1: it is precisely because of these vibrations that occur at higher and higher amplitudes that materials
expand when they get hotter.
nerdy aside 2: if we define temperature as the presence of vibrations like these - the temperature of "absolute" zero is NOT, however, the absence of all vibrations. There is always a single lowest energy vibration that can take place in a molecule - a "zero energy" vibration is not allowed to exist. so at "absolute zero" all the molecules in the material are vibrating in that single lowest energy mode. Absolute zero being -459 F.