So now I think I want to change things up a bit. In the past I have been talking about cases where the heating element inside the oven is just blasting away at its full output, and trying to give insight on how the temperature of the oven settles in on a certain temperature, and why that temperature might be too low (because the outside wall is allowed to get hot enough that the loss of heat there is equal to the full output of the heating element .... and so the heating element just cant keep up enough to raise the temperature any further.
So .... lets start talking about insulation. so far I have said lets consider the thermal conductivity of the in wall is constant, and said that a greater temperature gradient in the wall coincided with a greater heat flow through the wall (which was convenient at the time). But .... lets go back to that equation, and instead ask - if for a given value of Q (heat input from the heating element) - how does a changing thermal conductivity affect the temperature gradient. so starting with:
Q = - k * dT/dx
Do just a little bit of algebra to get:
dT/dx = -Q / k
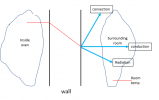
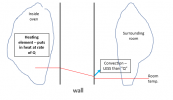
IN other words, if the wall is a better insulator (thermal conductivity k is smaller), then for a given value of Q (heat input from the heating element) the temperature gradient in the wall is BIGGER. This is how we intuitively think about how insulation works: when it is cold outside your house, good insulation in the walls keeps the inside walls nice and warm. So, as an intermediate step, lets go back to an earlier diagram where we were able to "magically" fix the outside temperature of the wall at room temperature .... but instead visualize how the changing thermal conductivity of the wall affects the temperature profile in the wall for the case where the heat input from the heating element, Q, is constant:

So ... even though we now loose the "temperature slope is proportional to the heat flux Q" .... this still kind of makes intuitive sense: a poorer insulation in the wall gives rise to a lower temperature inside the oven (again, the heating element is running full tilt ... .and if the heating element is not strong enough, then the temperature inside the oven can not get hot enough).
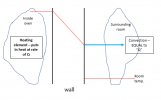
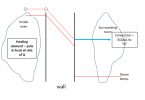
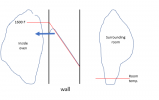
But now .... and Im sorry about this .... I need to add what might be the most confusing thing about this. NOW, lets take the case where we fix the outside temperature at room temperature, And we use a heating element with power sufficient to overcome the bad insulation in the wall, AND we use a PID to control the inside temperature of the oven, say to 1600F. This situation looks visually like this:

So what should look weird about this is that for very different wall thermal conductivities,
the temperature slope in the wall is the same. How can that be given everything I said before???? Well .... look again at the diagram above, and note that
I have not said anything about fixing Q. In this case, we have
fixed the value of the temperature gradient dT/dx by using the PID to pin the inside temperature, and have also said we are magically controlling the outside temperature to be room temperature. We have also said we are defining the value of K (thermal conductivity). Now Go back to the equation for heat flux through the wall:
dT/dx = -Q / k
The only way for dT/dx to be unchanged as k is varied is for Q to change (Q is bigger for larger k, and smaller if k is smaller - i.e. the ratio between Q and k has to be unchanged.). A
lthough you will not see it unless you look closely at your oven running - this is taken care of by your PID! If thermal conductivity is large (large k / poor insulator), then the PID will cycle the heating element on more often. if the thermal conductivity is small (small k / good insulator), then the PID will cycle the heating element on infrequently. sorry - in this case you just need to do the math to see where the heat flux Q settles.
This is why I tend to really like "thought experiments" - they get away from the math. For example, for a really, really efficient insulating wall (like my earlier 100 mile thick wall), once the inside of the oven is up to temperature - even though you still have that temperature gradient in the wall - heat loss through the wall to the outside is really, really small - and the heating element basically needs to put zero heat into the oven for the temperature there to say basically constant (this is - after all - exactly how a really good vacuum thermos works to keep your coffee hot!).
In these cases of thick walls / small values of thermal conductivity -
a powerful heating element is useful for getting the oven up to temperature quickly - but are not needed to maintain the temperature once it has been reached. But remember .... for steady state (fully soaked) conditions, we did not, and never will loose, the requirement that the heat input from the heating element, Q, must be the same as the total heat lost from the outside of the oven.
Please let me know affirmatively if this makes sense, or whether I have completely lost you. Because ... the next (and I think second to last) step is to bring back convective loss at the outside of the oven and allow the temperature there to change and settle in on where it needs to be for Q to be constant everywhere.....