- Joined
- Jun 3, 2019
- Messages
- 2,714
Next post:
What gets in the way of filling the porespace with resin? – WATER
A funny thing happened on the way to the (knife) scales:
Several weeks ago I scored a bunch of basically dried black walnut from a local guy who sources it from southern MN farmland, and turns it in to slab tables (they are gorgeous!). He has a supply of “ends” which are just perfect for knife scales. Although these were “kiln dried” they had been sitting in a pretty uncontrolled garage. So … after cutting the slabs down to 2x2” blocks, some of my “leftovers” were ½” slabs (perfect for the small “dinner” knives I am making for my wife). I decided to see if I could “push” them to total dryness by putting them in to a food dehydrator I have (145F max temp, circulated) – I also wanted to see just how dry they had stayed. Here they are – just a few small pieces:
 v
v
(I’ll explain the “138” in a little bit).
Before I put them into the dehydrator, I weighed them. They were 145g (according to my kitchen scale, above). It is not perfect, but probably accurate enough for current purposes. I DO have access to a laboratory scale accurate to 0.0001g – might pull it out later if seems necessary . Anyway, I put these babies into that food dehydrator (think of it as a small but very active “drying box” at 145F). After weighing once in a while as things went, they lost weight, and leveled out (i.e. lost no more weight) after 24 hours at 135g. (the "138" was measured after about 4 hours after taking them out of the dehydrator. they had pulled water out of the air and regained that weight all on their own)
Ok, they lost 10g of water. 10g/145g = ~7% moisture initially. Seems reasonable.
But wait – this is 7% by weight – and water is more dense than (dry) wood. So – what does this LOOK like?
10g of water is 10mL of water. So – this is what that volume of water looks like next to those slabs of wood:

That is a LOT of water relative to the volume of the wood it came out of. I do not know about you – but faced with the visual reality (not just some number like “6%”, I was rather taken aback. Where does all this water go in the wood?
Ordinarily with something like this, we speak about adsorption of water to the surface (or inner surfaces) of something (and yes, the word is “adsorption”, not “absorption”). But adsorption to something is a molecularly thin surface layer – not a lot. However, there is a phenomenon called “capillary condensation”, where liquid water condenses and fills very small spaces (capillaries), even at pretty high temperatures (the explanation for why this is comes from statistical thermodynamics – think lots of big hairy equations … so lets not go there here. Suffice it to say that this is an observed and measurable phenomenon). Also suffice it so say that there is a lot of journal data out there to confirm that capillary condensation is a very active mechanism in moisture retention in wood.
If you have a lot of capillaries, you can hold a LOT of water. The other aspect of capillary condensation is that it is very, very, highly biased towards putting that water into liquid form in the capillaries. Even with very low ambient humidity, the forces involved will “pull” the water out of the air and cause it to condense in those capillaries. This mechanism is, in fact, exactly how many very effective desiccants work. The other thing to know is that the amount of liquid water in the capillaries exists in an equilibrium with the ambient humidity, with the balance of that equilibrium being dependent on temperature (the lower the temperature the more capillary condensation, the higher the temperature the less the capillary condensation). This water will not dry out with time – in fact the wood will actively “pull” water into it, even with very low ambient humidities.
So – going back to our “model pore”, water in the porespace looks something like this:
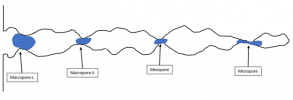
Where the blue areas are meant to be condensed (liquid) water.
Three points:
1) when you pull a vacuum and pull the air out of the porespace, you will not remove very much of the water: the air will just kind of push the water aside to the walls where it will stay, and then when the air stops rushing by, the water will just “pool up” again in the small pores (remember – the forces creating these liquid water pools are extremely strong).
2) When you try to force acrylic resin into the porespace, you might succeed in “pushing” the water deeper into the pores, but you will not remove it. Ultimately, the volume taken up by this water is pore volume that the resin will not fill.
3) Remember there is a LOT of this liquid water, filling a lot of the porespace. Not good for filling as much of the porespace with resin as you can.
So just how do you go about getting rid of that liquid water in the wood? One way to look at this is from information in the Wood Handbook ( https://www.fpl.fs.fed.us/documnts/fplgtr/fpl_gtr190.pdf ) which gives some cool information on water content versus temperature and humidity. One figure they present on equilibrium moisture content is this:

So – you can reduce moisture content by varying either ambient temperature or ambient humidity. Accurately controlling ambient humidity, especially to very low values is HARD (I have done it while characterizing desiccants – it takes a $15-20k piece of equipment, and a lot of tuning of the controllers). So for our practical purposes, that leaves only TEMPERATURE. Lets pick on the 50% RH data (the handbook also gives numerical values). The relationship at 50% RH between equilibrium moisture content and temperature looks like this:
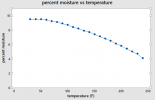
Look closely at that curve. Even if you heat the wood to 240F, you are only going to be able to remove about half of the residual moisture, no matter how long you heat it for.
Now – these are more or less average values. The actual values will depend on species – but this should be enough to make the points. And the points are:
1) If you do nothing to remove moisture in the wood before trying to impregnate with resin, there will be a LOT of liquid water in the pores, and that liquid water will block the resin from filling a lot of the porespace.
2) The only practical way of removing that water is to heat the wood – the hotter the better (without burning the wood of course). Even then, you will never get all of the water out.
3) Capillary condensation is a powerful and fast mechanism. Once you remove the wood from the oven (or dryer) it will immediately start pulling water out of the air. A lot of it. Quickly. So protect it while it cools (zip loc bags with air removed, or better yet vacuum sealed food storage bags. (do NOT put the wood into the resin while hot - the heat will start the resin curing before you can get it into the pores)
One other thing: moisture meters, especially the two-prong conductivity meters, become very inaccurate at low values of moisture (the reason comes from something called “percolation theory” – again coming from the petroleum industry – really cool (you can demonstrate it with M&M’s ) – but that is way beyond the current topic). So … if you want to track the moisture content of your drying wood as you heat it, get a digital scale and let weight loss over time guide you. Timeframes for typical scales or 2x2 blocks will likely (just based on my recent experience) be measured in days. K&G does bake their stuff prior to impregnating with resin, and they use a moisture meter to judge degree of dryness.
What gets in the way of filling the porespace with resin? – WATER
A funny thing happened on the way to the (knife) scales:
Several weeks ago I scored a bunch of basically dried black walnut from a local guy who sources it from southern MN farmland, and turns it in to slab tables (they are gorgeous!). He has a supply of “ends” which are just perfect for knife scales. Although these were “kiln dried” they had been sitting in a pretty uncontrolled garage. So … after cutting the slabs down to 2x2” blocks, some of my “leftovers” were ½” slabs (perfect for the small “dinner” knives I am making for my wife). I decided to see if I could “push” them to total dryness by putting them in to a food dehydrator I have (145F max temp, circulated) – I also wanted to see just how dry they had stayed. Here they are – just a few small pieces:
 v
v(I’ll explain the “138” in a little bit).
Before I put them into the dehydrator, I weighed them. They were 145g (according to my kitchen scale, above). It is not perfect, but probably accurate enough for current purposes. I DO have access to a laboratory scale accurate to 0.0001g – might pull it out later if seems necessary . Anyway, I put these babies into that food dehydrator (think of it as a small but very active “drying box” at 145F). After weighing once in a while as things went, they lost weight, and leveled out (i.e. lost no more weight) after 24 hours at 135g. (the "138" was measured after about 4 hours after taking them out of the dehydrator. they had pulled water out of the air and regained that weight all on their own)
Ok, they lost 10g of water. 10g/145g = ~7% moisture initially. Seems reasonable.
But wait – this is 7% by weight – and water is more dense than (dry) wood. So – what does this LOOK like?
10g of water is 10mL of water. So – this is what that volume of water looks like next to those slabs of wood:

That is a LOT of water relative to the volume of the wood it came out of. I do not know about you – but faced with the visual reality (not just some number like “6%”, I was rather taken aback. Where does all this water go in the wood?
Ordinarily with something like this, we speak about adsorption of water to the surface (or inner surfaces) of something (and yes, the word is “adsorption”, not “absorption”). But adsorption to something is a molecularly thin surface layer – not a lot. However, there is a phenomenon called “capillary condensation”, where liquid water condenses and fills very small spaces (capillaries), even at pretty high temperatures (the explanation for why this is comes from statistical thermodynamics – think lots of big hairy equations … so lets not go there here. Suffice it to say that this is an observed and measurable phenomenon). Also suffice it so say that there is a lot of journal data out there to confirm that capillary condensation is a very active mechanism in moisture retention in wood.
If you have a lot of capillaries, you can hold a LOT of water. The other aspect of capillary condensation is that it is very, very, highly biased towards putting that water into liquid form in the capillaries. Even with very low ambient humidity, the forces involved will “pull” the water out of the air and cause it to condense in those capillaries. This mechanism is, in fact, exactly how many very effective desiccants work. The other thing to know is that the amount of liquid water in the capillaries exists in an equilibrium with the ambient humidity, with the balance of that equilibrium being dependent on temperature (the lower the temperature the more capillary condensation, the higher the temperature the less the capillary condensation). This water will not dry out with time – in fact the wood will actively “pull” water into it, even with very low ambient humidities.
So – going back to our “model pore”, water in the porespace looks something like this:

Where the blue areas are meant to be condensed (liquid) water.
Three points:
1) when you pull a vacuum and pull the air out of the porespace, you will not remove very much of the water: the air will just kind of push the water aside to the walls where it will stay, and then when the air stops rushing by, the water will just “pool up” again in the small pores (remember – the forces creating these liquid water pools are extremely strong).
2) When you try to force acrylic resin into the porespace, you might succeed in “pushing” the water deeper into the pores, but you will not remove it. Ultimately, the volume taken up by this water is pore volume that the resin will not fill.
3) Remember there is a LOT of this liquid water, filling a lot of the porespace. Not good for filling as much of the porespace with resin as you can.
So just how do you go about getting rid of that liquid water in the wood? One way to look at this is from information in the Wood Handbook ( https://www.fpl.fs.fed.us/documnts/fplgtr/fpl_gtr190.pdf ) which gives some cool information on water content versus temperature and humidity. One figure they present on equilibrium moisture content is this:

So – you can reduce moisture content by varying either ambient temperature or ambient humidity. Accurately controlling ambient humidity, especially to very low values is HARD (I have done it while characterizing desiccants – it takes a $15-20k piece of equipment, and a lot of tuning of the controllers). So for our practical purposes, that leaves only TEMPERATURE. Lets pick on the 50% RH data (the handbook also gives numerical values). The relationship at 50% RH between equilibrium moisture content and temperature looks like this:

Look closely at that curve. Even if you heat the wood to 240F, you are only going to be able to remove about half of the residual moisture, no matter how long you heat it for.
Now – these are more or less average values. The actual values will depend on species – but this should be enough to make the points. And the points are:
1) If you do nothing to remove moisture in the wood before trying to impregnate with resin, there will be a LOT of liquid water in the pores, and that liquid water will block the resin from filling a lot of the porespace.
2) The only practical way of removing that water is to heat the wood – the hotter the better (without burning the wood of course). Even then, you will never get all of the water out.
3) Capillary condensation is a powerful and fast mechanism. Once you remove the wood from the oven (or dryer) it will immediately start pulling water out of the air. A lot of it. Quickly. So protect it while it cools (zip loc bags with air removed, or better yet vacuum sealed food storage bags. (do NOT put the wood into the resin while hot - the heat will start the resin curing before you can get it into the pores)
One other thing: moisture meters, especially the two-prong conductivity meters, become very inaccurate at low values of moisture (the reason comes from something called “percolation theory” – again coming from the petroleum industry – really cool (you can demonstrate it with M&M’s ) – but that is way beyond the current topic). So … if you want to track the moisture content of your drying wood as you heat it, get a digital scale and let weight loss over time guide you. Timeframes for typical scales or 2x2 blocks will likely (just based on my recent experience) be measured in days. K&G does bake their stuff prior to impregnating with resin, and they use a moisture meter to judge degree of dryness.



 790D384B-7241-49D6-87E8-2F09F4F6551C
790D384B-7241-49D6-87E8-2F09F4F6551C